This database was compiled at the request of the European Commission and contains more than 900 terms in 35 (!) languages.
The terminology covers:
- dosage forms,
- routes and/or methods of administration,
- presentations,
- packaging, closures and delivery devices…
… for medicinal products for both medical and veterinary use.
Standard Terms terminology is used in marketing authorizations, labeling (including summaries of product characteristics (SmPCs)), electronic communications and adverse-event reporting.
❗️ To access the database, you need to register at the website (for free).
1️⃣ Click “Open resource” here and go to EDQM registration website using a button in the end of the page:

2️⃣ Register here. (It’s simple and quick. You will see additional explanations along the way.)
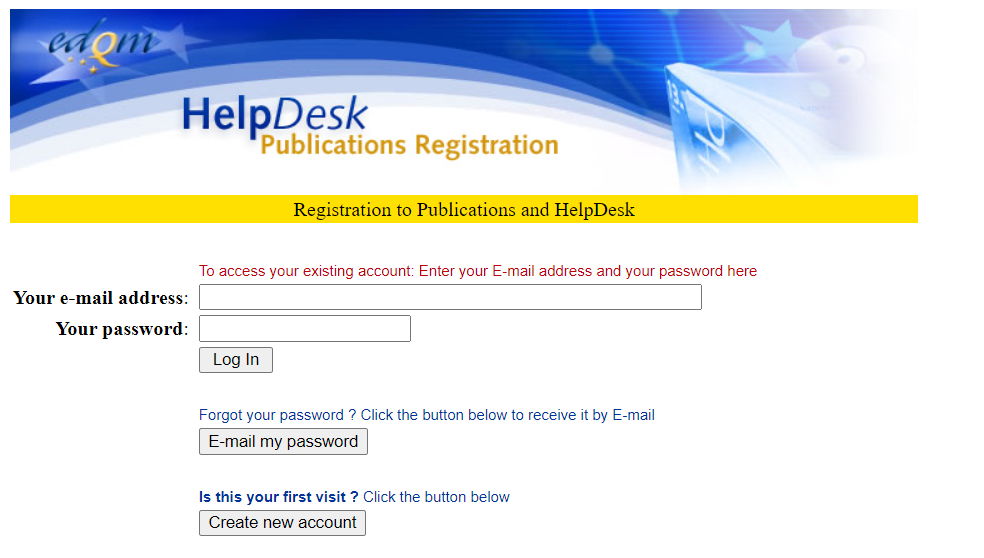
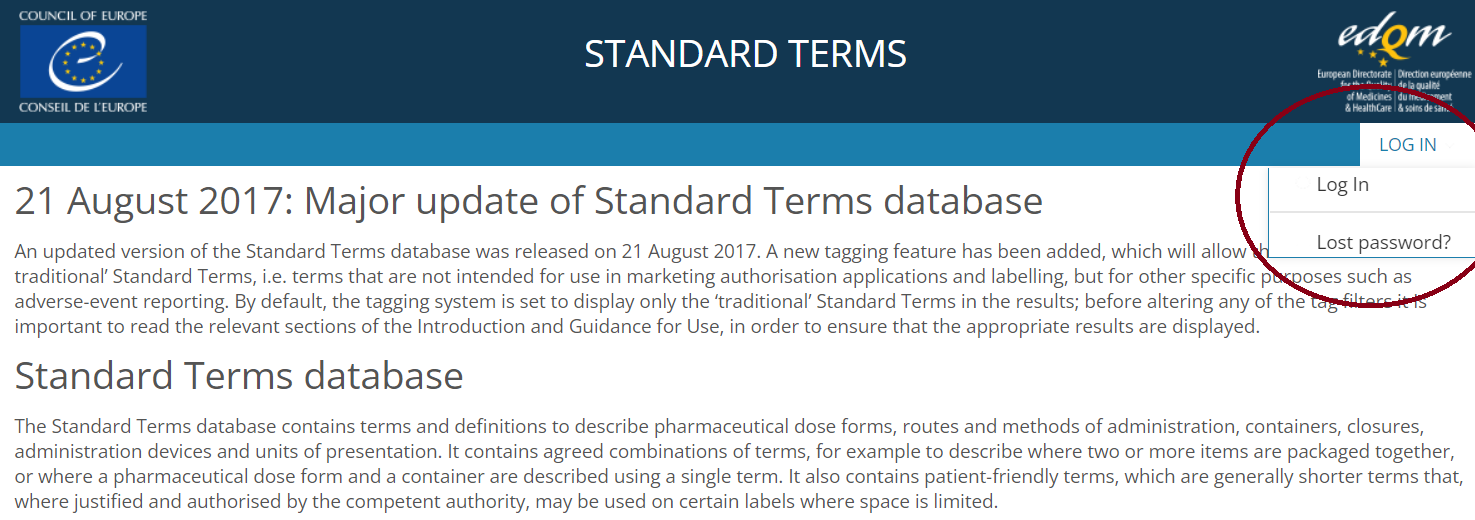
3️⃣ You will receive an email with your access details and a link to the database (https://standardterms.edqm.eu). Go to the database and log in.
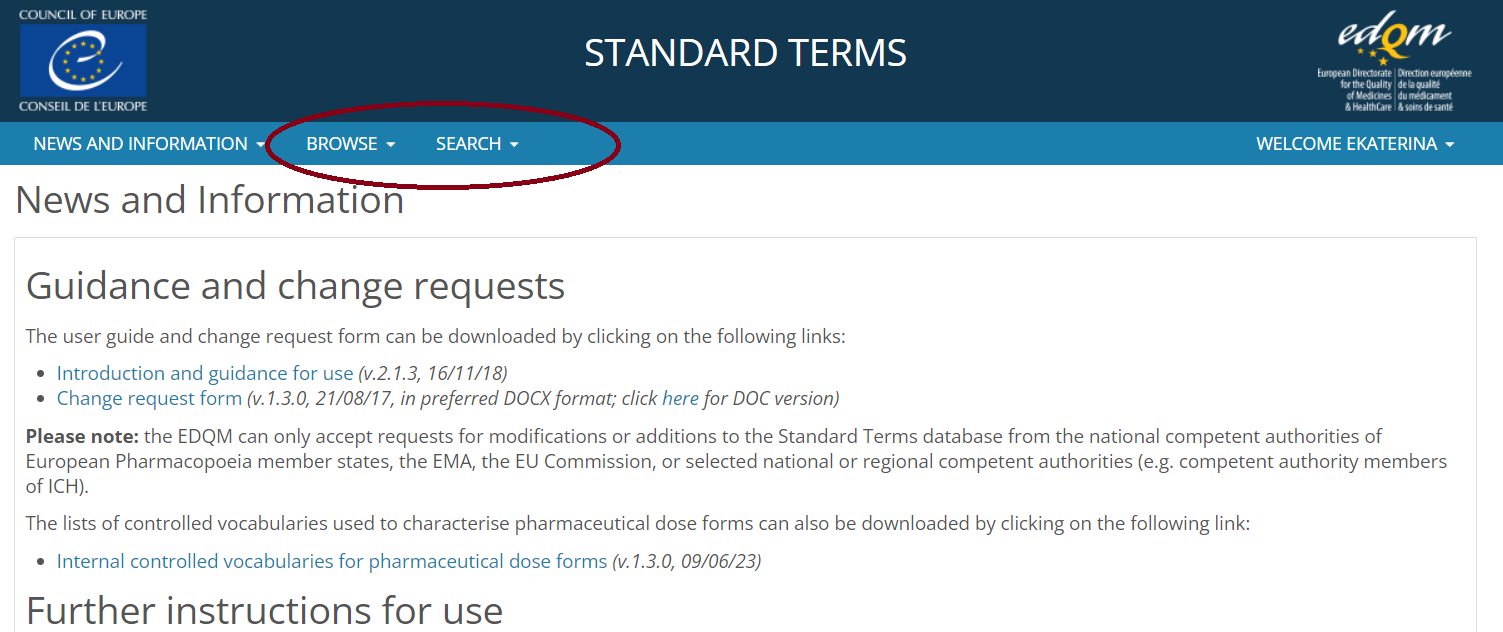
4️⃣ You’re all set! Browse the database by categories or use the search functionality.




 Copy link
Copy link
 E-mail
E-mail
 LinkedIn
LinkedIn
 Facebook
Facebook
 Telegram
Telegram
 WhatsApp
WhatsApp

